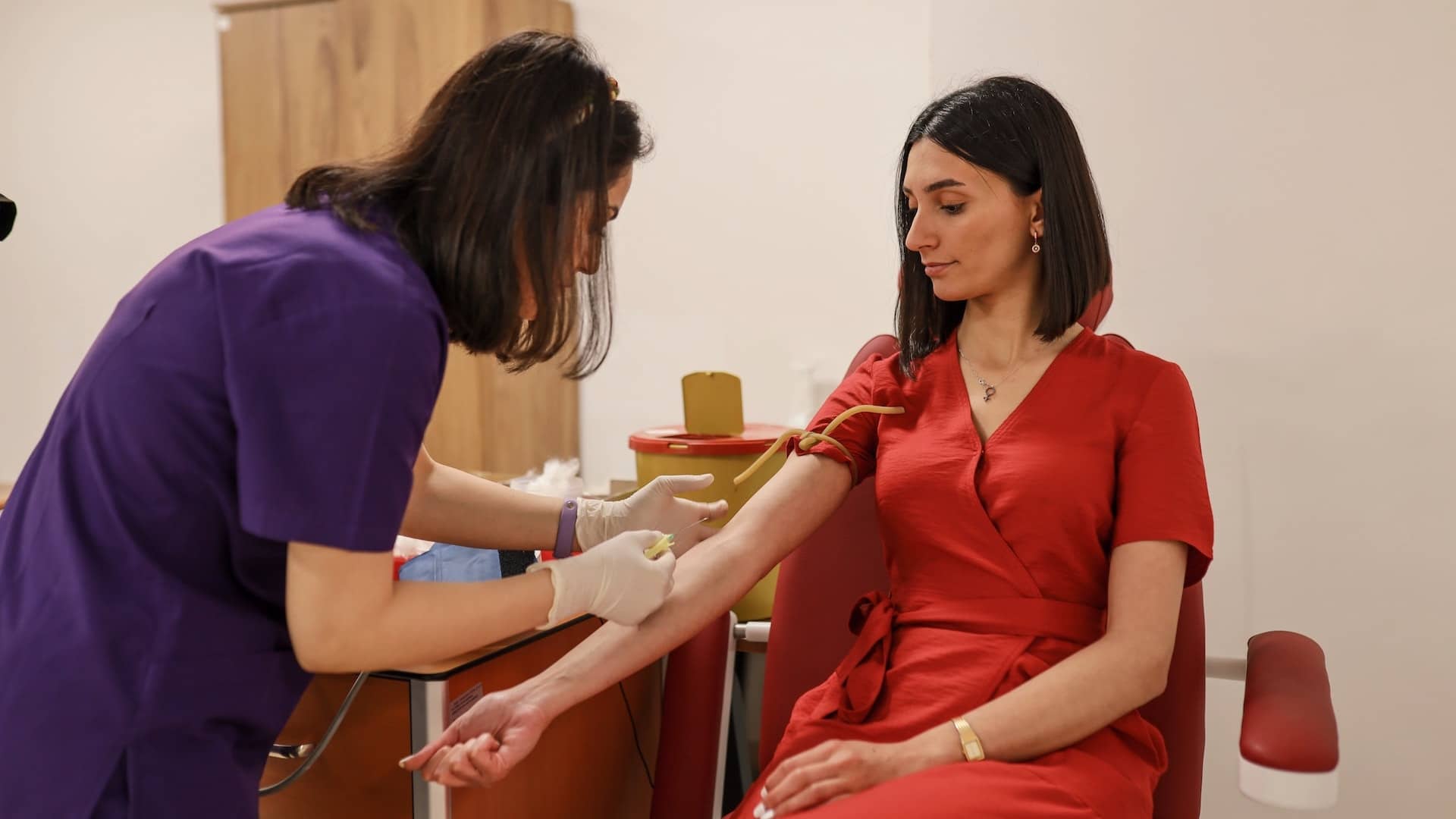
Scientists from MIT designed a microfluidic device to capture and count circulating plasma cells in small samples of blood. This technique can only be carried out through routine blood drawing, which is expected to reduce the pain of patients' myeloma testing.
The microfluidic device designed by MIT scientists can separate plasma cells from blood instead of bone marrow. The research was published in Scientific Reports, a sub journal of Nature.

Maybe many people think it is nothing strange, but for patients with multiple myeloma, bone marrow biopsy is a very painful thing. It will be of great significance for patients to replace bone marrow biopsy with blood test, not only to reduce pain.
Multiple myeloma is a plasma cell cancer. Plasma cells are produced in the bone marrow and produce antibodies to help fight infection. When plasma cells become cancer cells, they will produce abnormal proteins. After the cells are established in the bone marrow, they will eventually penetrate into the blood. The typical diagnosis of multiple myeloma is made through bone marrow biopsy. A needle is inserted near the patient's hip bone to aspirate bone marrow samples. Clinicians can determine whether they are cancer cells by separating plasma cells from bone marrow.
Scientists from MIT designed a microfluidic device to capture and count circulating plasma cells in small samples of blood. Microfluidic cell capture is designed based on CD138 antigen. CD138 is highly expressed on plasma cells, which can be used for quantitative analysis of circulating plasma cells (CPC) rare in blood and subsequent fluorescence analysis. This technique can be carried out through routine blood drawing, which is expected to reduce the pain of patients' myeloma testing.
This microfluidic chip adopts ingenious fishbone channels and repeated V-shaped groove etching, which is very similar to fishbone. Such grooves enable any fluid (blood sample) to generate vortex when passing through the microchip, rather than directly passing through. The cells in the fluid thus have a high chance of contacting the inner wall of the device. Inside the microfluidic device, the inner wall of the microchip is coated with specific molecules, which can identify and attract the cells we want. The main design of the device is from George Whitesides team of the chemistry department of Harvard University.
One of the co authors of the article, Dr. Rohit Karnik from MIT and his team used the microfluidic fishbone design to capture circulating plasma cells in the blood. They coated CD138 antibody in microchip pore. The team then used 1ml of blood sample to pass through the microfluidic device. The antibody in the fishbone pore would capture the plasma cells in the blood, allowing other blood components to pass through the device.
When plasma cells are separated through a microfluidic device, researchers will count the cells and characterize them.
The cell capture rate displayed in the model is about 40-70%, and it can be identified at a low level where the number of CPC in 1ml blood is less than 10, which is difficult to achieve with existing technology.
In bone marrow samples, the plasma cell technology based on microfluidics showed excellent correlation with the data analyzed by flow cytometry. It shows that the design of microfluidics can achieve high sensitivity and accuracy similar to precision instruments.
In the peripheral blood samples, the device detected 2-5 CD138+cells per milliliter of healthy donor blood at the baseline. In the samples of multiple myeloma remission donors, the data increased significantly, which was 20-24 CD138+cells, while in the donor blood displayed by multiple myeloma diseases, the data reached 45-184 CD138+cells.
These results indicate that cell capture based on this microfluidic device has a great possibility to replace bone marrow biopsy, which can not only be used to judge diseases, but also be used to evaluate the effectiveness of treatment schemes.
It is worth mentioning that the team pointed out that the circulating plasma cells of patients in remission stage showed higher counts than those of healthy donors. These patients are often shown as normal indicators in routine blood tests. Karnik said that the new device may acquire more subtle information about the patient's disease status, even in the remission period.
This is of great significance for patients undergoing chemotherapy.
"The process of traditional tissue biopsy is very painful, followed by complications such as potential infection, and often can only be carried out in central hospitals, requiring patients to travel long distances," said Mohammad Qasaimeh, a former MIT postdoctoral fellow, "Plasma cells captured from blood samples can be used as a kind of liquid biopsy, which can be carried out as often as needed in clinical practice, and can be used as a diagnostic and prognostic test for therapeutic effects after chemotherapy. In addition, cells captured can be used for drug testing, so they can be used as personalized medical tools."
MIT's microfluidic device has the precision and sensitivity comparable to that of professional instruments. Moreover, it can provide unique indication information for treatment tracking and prognosis that are difficult to be achieved by existing detection methods, and the captured plasma cells can be used for drug development and treatment screening. In addition, this blood sample based biopsy is expected to not only avoid the pain of bone marrow biopsy for patients with myeloma, but also avoid possible infection and complications of bone marrow biopsy.
We expect that this powerful new detection method can be applied to the clinic as soon as possible to help more patients with myeloma improve their quality of life and treatment.