Recently, the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) accused Moderna of withholding data during the approval process for its new bivalent crown vaccine, and several FDA and CDC advisors said, "We are outraged and disappointed by this action by Moderna! In order to obtain a $5 billion taxpayer order, Moderna concealed factual data that 3.2 percent of study participants who received the bivalent vaccine were infected, while only 1.9 percent of those who received the monovalent vaccine were infected. Both Moderna and the FDA have unshirkable responsibility in this matter." Currently, spokespeople for Moderna and the FDA disagree on who is responsible for the missing data.

Was the omission of critical data intentional or unintentional?
For new vaccines to be approved, both the FDA and CDC must convene their advisory committees and make a presentation to the advisory panel. This objective review panel will then vote on whether to allow the new vaccine to pass approval. These independent advisors include infectious disease experts and vaccinologists from Stanford University, the University of Pennsylvania and Harvard University, but they have now expressed concerns about some of the information Moderna presented to them during discussions about the approval of its bivalent vaccine.
At an FDA meeting in June and a CDC advisory panel meeting in September, Moderna bivalent vaccine data showed that the new bivalent vaccine was more effective than the monovalent. These results were based on laboratory tests: blood taken from bivalent vaccine recipients was exposed to Omicron and then compared to samples from people who received the monovalent vaccine to measure the effectiveness of each vaccine in fighting antibodies to the new coronavirus. However, other data from the same study, which reflected the actual infection, were not presented to the panel. The concealed data showed that 3.2% of study participants who received the bivalent vaccine were infected. In comparison, only 1.9% of participants who received the monovalent vaccine were infected. However, the FDA's 22-page briefing document to the consultants made no mention of this infection data, nor did Dr. Jerry Weir, director of the Division of Viral Products in the FDA's Office of Vaccine Research and Review, mention the infection data in his presentation to the consultants.
Spokespeople for Moderna and the FDA disagreed on who was responsible for the missing data, and Moderna spokesperson Christopher Ridley said: "Moderna shared the infection data with the FDA prior to the FDA panel's June meeting, after the FDA requested updated data from ongoing studies. infection data. The study was publicly posted online as a preprint on June 25, three days before the panel met." However, FDA spokesman Michael Felberbaum said, "The FDA received the preprint less than a day before the advisory committee meeting, at which point it was too late for the preprint to be reviewed and incorporated into the agency's meeting materials."
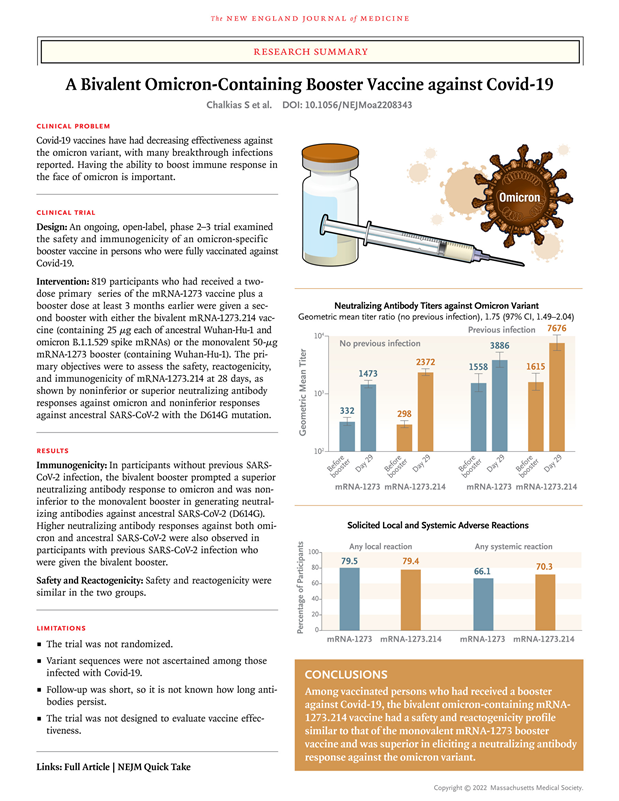
Moderna bivalent vaccine clinical phase 2/3 data
Moderna's senior vice president, Dr Jacqueline Miller, was accused of using "carefully selected" materials in a presentation to CDC consultants in September. Dr. Miller cited disease incidence in those with and without evidence of prior infection, which highlights the effectiveness of the bivalent vaccine in a more positive light, but did not mention the incidence of neo-coronavirus in those who had not previously been infected. incidence rates for hundreds of study participants, as these data show that monovalent vaccination is significantly more effective in preventing infection.
FDA needs credibility and strives for transparency of data

Dr. Eric Rubin, a member of the FDA's Vaccine Advisory Committee, said, "Our consultants are not a bunch of kids, not so easily fooled, and they know how to interpret these results." The six FDA and CDC advisers said the infection data that Moderna withheld would not change how they voted because the data had limitations, but that Moderna should still present the full data to the advisers.
The advisors considered the infection data that Moderna did not provide to them to be important for three main reasons: first, the decisions they make are potentially influential and all available data should be on the table for evaluation if Americans are to receive these vaccines; second, advisory committee meetings are streamed live online and regulators around the world will refer to this information to make decisions about their country's vaccines decisions; third, transparency is important to the public, and it is about the credibility of the FDA. The public does not see the conversations between FDA officials or between agency officials and pharmaceutical company executives, but they do have access to the minutes of the advisory panel meetings. The public would only be reassured if all data, not just some, were discussed at advisory meetings.
Dr Arnold Monto, professor of epidemiology at the University of Michigan School of Public Health and acting chair of the FDA advisory panel, said: "Moderna should always be completely transparent about the data and should not ignore them. Although it is only early data, the results show that we need to study them and measure their value." Dr Pablo Sanchez, a member of the CDC panel, said, "The Advisory Committee on Immunization Practices has said that if data is reviewed as part of a study, it should be submitted to consultants before they make a decision."
FDA spokesman Michael Felberbaum said, "The FDA received a preprint less than a day before the advisory committee meeting. As a result, the information was not provided in sufficient time to be included in the FDA meeting materials, and typically the FDA only discusses data that the agency has had an opportunity to substantially review at advisory committee meetings. The neo-crown vaccine remains the best defense against neo-crown severe disease and an upgraded vaccine may help provide better protection against the currently prevalent variant of the virus. The FDA has been as transparent as possible throughout the pandemic with regard to the process and decision making for the neo-crown vaccine, and Moderna could have chosen to submit the data prior to the FDA advisory committee meeting."
Bivalent mRNA vaccine: inability to boost BA.4 and BA.5 neutralising antibody titres
Given the ability of mRNA technology to respond rapidly to mutant strains, bivalent vaccines were created. in January 2022 and February 2022, Pfizer-BioNTech produced a bivalent vaccine containing 15 μg of mRNA against the ancestral New Crown strain and 15 μg of mRNA against BA.1. Moderna used 25 μg of mRNA against the same two virulent strains.
On June 28, 2022, researchers from Pfizer-BioNTech and Moderna presented data on their bivalent vaccine to the FDA's Advisory Committee on Vaccines and Related Biological Products. The results were disappointing: the bivalent booster resulted in neutralising antibody levels against BA.1 that were only 1.5 to 1.75 times those of the monovalent vaccine. Experience suggests that this difference is unlikely to be clinically meaningful. In the meantime, BA.1 is no longer circulating in the US and has been replaced by the more immune evasive and infectious Omicron subvariant. However, winter had arrived, the spread of Omicron had accelerated, and in response to these immune evasive strains, the FDA Advisory Committee decided to pass a review.
A series of policies followed. on June 29, 2022, the day after the advisory committee meeting, the Biden administration agreed to purchase 105 million doses of Pfizer-BioNTech bivalent vaccine for $3.2 billion. on July 29, 2022, the administration agreed to purchase 66 million doses of Moderna's bivalent vaccine for $1.74 billion and intends to make both vaccines available in the fall and winter. On September 1, 2022, the FDA revoked the emergency use authorization for the monovalent vaccine booster and the CDC recommended the bivalent vaccine booster for everyone 12 years of age or older.On October 12, 2022, the CDC expanded this recommendation to include all individuals 5 years of age or older. However, data from humans, including immunogenicity data, were not available at this time.
On 24 October 2022, David Ho and colleagues published a study of neutralising antibody levels against BA.4 and BA.5 after receiving monovalent or bivalent booster doses. They found "no significant difference in neutralisation of any of the new crown variants" between the two groups. A day later, Dan Barouch and colleagues published the results of a similar study, finding "little difference in BA.5 neutralising antibody titres between the monovalent and bivalent mRNA booster groups." Barouch and colleagues also noted no significant differences in CD4+ or CD8+ T-cell responses between monovalent and bivalent plus vaccinated participants. Neither team found that the bivalent vaccine triggered a better immune response.
Six months after the FDA advisory meeting, Moderna has yet to release data from another randomised phase 3 trial with 3,000 participants that compared infections in bivalent vaccine recipients to those in monovalent vaccine recipients. Moderna's bivalent vaccine was made available to all Americans over the age of 12 at the end of August, but vaccination rates were very low. According to the FDA, only 15.4% of the US population received the bivalent vaccine, compared to about 70% of people who received the monovalent vaccination. Even among the highest risk age group (65 years and older), only 38% chose to receive the bivalent vaccine. This low vaccination rate has been attributed to concerns about the lack of transparency in the vaccine development and approval process, and these recent allegations made by FDA advisors may further fuel this concern.