Recently, the US FDA granted priority review status to a new indication application for Enhertu (DS-8201) for the treatment of adult patients with unresectable or metastatic HER2 low expression breast cancer who have received 1 prior therapy. HER2 low expression is defined as immunohistochemistry 1+ or 2+ and negative in situ hybridization.
In April 2022, the FDA granted Enhertu breakthrough therapy designation for patients with unresectable or metastatic HER2-overexpressing breast cancer who have received prior systemic therapy or whose disease has recurred within 6 months of completing adjuvant chemotherapy. A decision on approval by the FDA is expected in the fourth quarter of this year.
Enhertu's application for the new indication is based on data from the Phase III DESTINY-Breast04 trial.
The trial showed that in patients with hormone receptor-positive, HER2 low-expressing metastatic breast cancer, the average length of time that patients treated with Enhertu remained disease-free was 10.1 months, while the average length of time that patients treated with chemotherapy remained disease-free was 5.4 months. The mean overall survival time for patients treated with Enhertu was 23.9 months, compared to 17.5 months for the chemotherapy group.
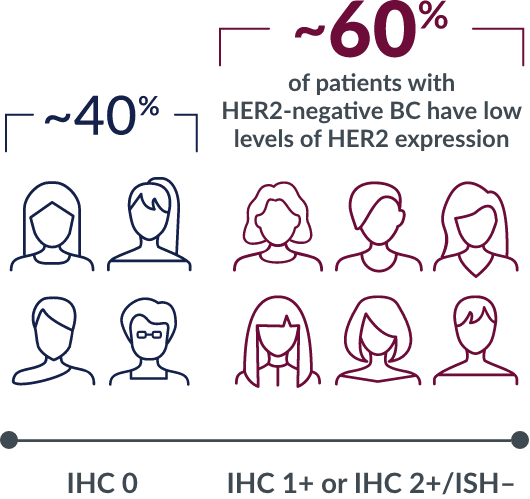
Trial details
The DESTINY-Breast04 trial enrolled 557 patients with unresectable or metastatic breast cancer who were hormone receptor positive or negative and had low HER2 expression and who had received previous 1st or 2nd line chemotherapy.
Patients were randomised in a 2:1 ratio into two groups, one receiving Enhertu at 5.4mg/kg every three weeks and the other treated with a chemotherapy regimen of the physician's choice, including capecitabine, eribulin, gemcitabine, paclitaxel or albumin paclitaxel.
Additional results of the trial were presented at the 2022 ASCO Annual Meeting and published in the New England Journal of Medicine. They are as follows:
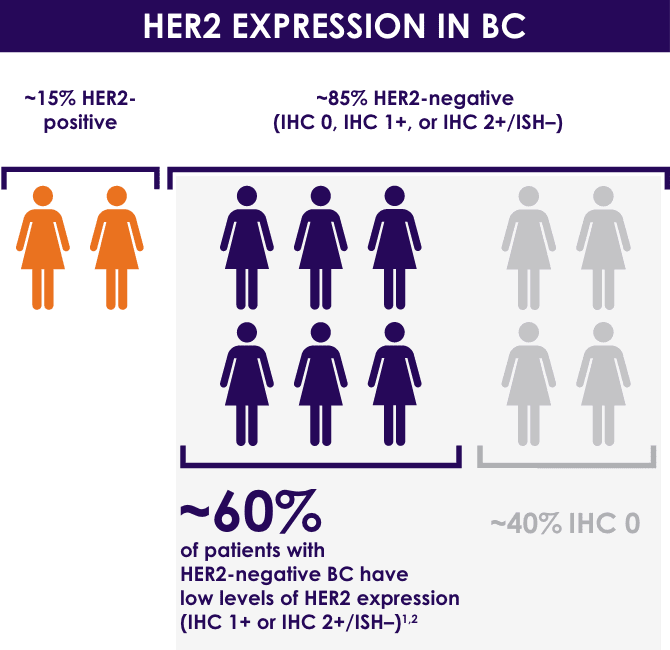
Among all patients who received the randomised grouping, the mean length of time to remain disease-free was 9.9 months in the Enhertu group and 5.1 months in the chemotherapy group. the mean overall survival time was 23.4 months in the Enhertu group and 16.8 months in the chemotherapy group.
The mean overall survival time was 18.2 months in the Enhertu group and 8.3 months in the chemotherapy group.
In hormone receptor-positive patients previously treated with CDK4/6 inhibitors, the mean length of time to remain disease-free was 10.0 months in the Enhertu group and 5.4 months in the chemotherapy group.
In hormone receptor-positive patients not previously treated with CDK4/6 inhibitors, the mean length of time to remain exacerbation-free was 11.7 months in the Enhertu group compared to 5.9 months in the chemotherapy group.
Safety and side effects
In terms of safety, 99.5% of patients in the Enhertu group experienced adverse reactions after treatment compared to 98.3% of patients in the chemotherapy group. fewer Grade 3 or higher adverse reactions were reported in the Enhertu group than in the chemotherapy group, 52.6% and 67.4% respectively.
Common treatment-related adverse reactions in the Enhertu and chemotherapy groups included nausea, fatigue, hair loss, vomiting, neutropenia and anaemia.

For more than two decades, only HER2-positive breast cancer patients have been able to benefit from HER2-targeted therapy, and the data from the DESTINY-Breast04 trial represent the first time that HER2-targeted therapy has shown a survival benefit in patients with low HER2 expression. If approved, Enhertu will redefine the classification and treatment of breast cancer, giving patients with low levels of HER2 expression in their tumours the opportunity to benefit from HER2-targeted therapy.






