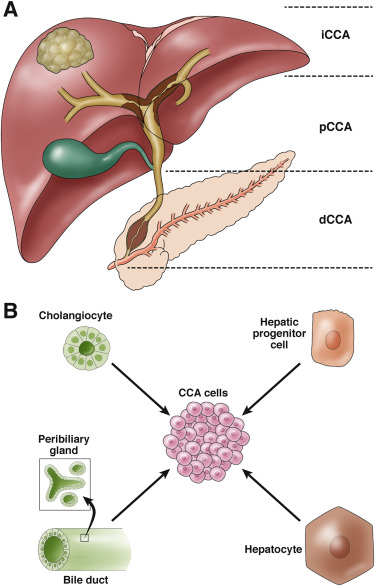
Cholangiocarcinoma is a rare but highly malignant tumour that occurs in the human bile duct system and has long had a poor prognosis. Now, researchers at University College London and University College London Hospital have identified potentially promising treatments that may radically improve outcomes for some patients with bile duct cancer in an international multicentre trial.
The phase II open-label clinical trial, led by researchers at University College London, found that patients with critically ill bile duct cancer could survive up to two years when treated with the drug Futibatinib.

Good data from the FOENIX-CCA2 clinical trial led to the US Food and Drug Administration (FDA) approving Futibatinib for marketing in September 2022, before the UK's National Institute for Clinical Excellence (NICE) had considered approving the drug.
The trial, conducted in 13 countries and supported by Taiho Oncology sponsorship, has had its results published in the prestigious New England Journal of Medicine.
Futibatinib targets a specific genetic mutation, called an FGFR2 fusion, which is found in around 14% of bile duct cancers.
About 300 people diagnosed with bile duct cancer (both cholangiocarcinoma and gallbladder cancer) each year in the UK will have this gene mutation, and in the US, about 8,000 patients will have it.
Until now, treatment options for cholangiocarcinoma were scarce and survival rates were poor, with the average length of survival for patients being only 12 months. Although this cancer is uncommon, the incidence is on the rise globally.
This international trial recruited 103 patients with bile duct cancer who had received at least one chemotherapy treatment but were resistant to it. The patients' tumours had been genetically analysed (molecular analysis) to check whether they had mutations in a specific set of FGFR genes. futibatinib, known as an FGFR-2 inhibitor, targets mutations in this gene, FGFR.
When patients are treated orally with Futibatinib, the results are very impressive. The drug was more effective in shrinking tumours compared to chemotherapy, with tumours shrinking by more than 40% compared to only 25% with chemotherapy. The drug has fewer side effects than chemotherapy.
Patients who received the treatment survived for up to two years, even though they had advanced cancer, and some had even tried up to five other treatments before entering the trial.
Professor John Bridgewater (University College London Institute of Cancer Research and University Hospitals London NHS Foundation Trust), European lead author and senior author of the paper, said: "These findings are transforming patient care. The drug could provide a more personalised, targeted treatment that targets only specific gene mutations in the cancer than chemotherapy."
"The benefits that patients are seeing in the trial are significant. It is important that patients with cholangiocarcinoma are screened for cancer to determine if they have this mutation and the huge impact this could have on treatment outcomes."
There are other FGFR inhibitors already in clinical use, including one called Pemigatinib, which has been approved for use by the UK's National Institute for Care and Health Excellence. However, the known existing FGFR inhibitors are very susceptible to drug resistance. However, laboratory tests have shown that Futibatinib can target FGFR mutations in a more specific way and may therefore be more effective than existing drugs.
Studies are now underway using FGFR2 inhibitors as first-line treatment instead of chemotherapy.
Professor John Bridgewater says: "The question that needs to be addressed in the research now is not whether patients should receive this treatment, but when it is better to receive it."
"Hopefully, this gene-driven targeted therapy will become the new normal in oncology. Personalised therapy has long been a buzzword in the cancer field, but this trial is more specific and real - demonstrating that Futibatinib can be of great benefit to a specific group of patients.
For more than a decade, little has changed in the treatment approach for those with inoperable bile duct cancer. Chemotherapy, while it may be effective for some people, is not effective for all. Some patients have been treated with several cycles of chemotherapy and may have endured many difficult side effects before finding out that it is not working well.
Now, for patients with bile duct cancer who are detected as having an FGFR2 fusion, Futibatinib is not only a more personalised treatment, but they know from the outset that the treatment will have positive effects, such as the promise of prolonged survival beyond standard chemotherapy and/or assertive supportive care. Moreover, as a tolerable oral therapy, patients are more likely to have quality-of-life advantages over conventional chemotherapy, including reduced hospital stays and not having to stay away from loved ones.






