Researchers at the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai have now identified a new treatment that promises to help cancer patients manage the side effects of bone marrow transplants better than current standard therapies, making the treatment more effective and safer. Results of the related Phase II clinical trial were presented at the American Society of Hematology (ASH) annual meeting in December.
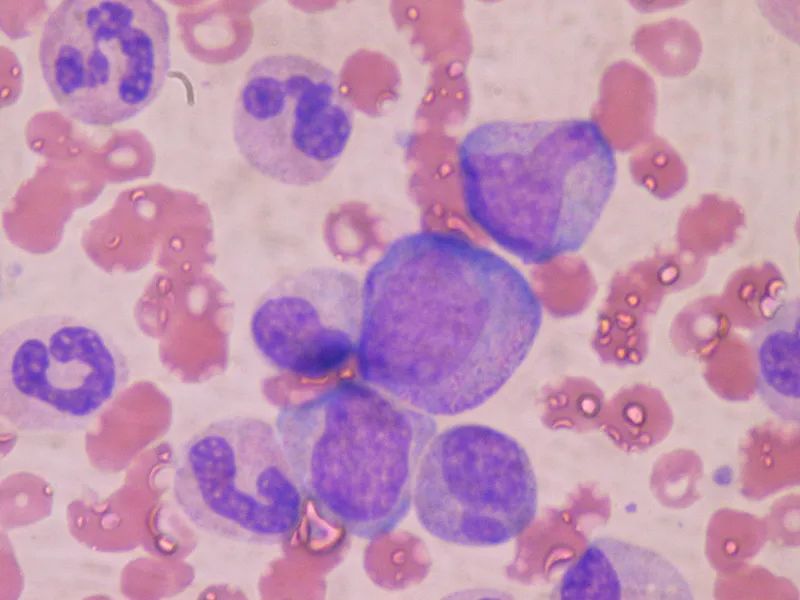
GvHD is a side effect seen in patients with blood cancers treated with donor bone marrow transplants. In this study, researchers screened patients with GvHD who might benefit from the new treatment by using a blood test developed at Mount Sinai.
"We know that steroids cause many complications in bone marrow transplant recipient patients who need treatment for GvHD, such as severe infections, bone and muscle damage, poor sleep, and poorer quality of life," said Aaron Etra, M.D., assistant professor of medicine, hematology and medical oncology at the Tisch Cancer Institute , who presented the study at the ASH meeting.
"Steroid-free treatment of GvHD would be an important step in improving the outcome of bone marrow transplantation therapy, but of course, it is not the best option for all patients. Giving patients the right personalised treatment by testing for biomarkers of GvHD is key to the success of this trial," added John Levine, MD, professor of medicine, haematology and medical oncology at the Tisch Cancer Institute, who is a co-senior author of the study. Alexandra Capellini, a student at Mount Sinai School of Medicine, is a co-author of the study.
GvHD occurs after a bone marrow transplant when cells from the donor attack a healthy organ in the recipient patient, and at this time, certain tissue proteins are released into the bloodstream; these proteins are used as biomarkers to quantify the severity of tissue damage.
Patients with low biomarker levels are usually treated well, but until then there is no good way to identify them effectively. A research laboratory at Mount Sinai Hospital is now able to quantify GvHD biomarkers in patients' blood samples within 30 hours, which is very helpful for GvHD patients who urgently need to start treatment quickly.
By comparing the results of the trial with control patients receiving standard steroid treatment, the study found that itatinib, a JAK1 inhibitor that suppresses the immune system, produced very good results for patients, that the treatment worked faster than steroid treatment and was as effective as steroid treatment over time, and that patients had equally good long-term outcomes.
Both itatinib and steroids were effective in 86% of patients, with a similar one-year survival rate of 88% for itatinib compared to 80% for steroids.
There was also a significant reduction in serious infections in patients treated with itatinib, possibly as a result of much reduced systemic steroid exposure.
"This is the first time that a real-time GvHD biomarker can be used to accurately identify low-risk patients whose treatment can be downgraded and steroid-free," said James L Ferrara, M.D., professor of medicine, hematology and medical oncology, Tisch Cancer Institute. "This newly developed biomarker approach allows us to take a meaningful step forward in helping patients improve their quality of life.






