A new study from the MD Anderson Cancer Center recently showed that in patients with stage 2-4 squamous cell carcinoma of the skin, immunotherapy prior to surgery resulted in substantial or even complete disappearance of tumours in 63.3% of patients.
The findings were reported at the annual meeting of the European Society of Medical Oncology and published in the New England Journal of Medicine.
The trial included 79 patients, all with operable stage 2-4 squamous cell carcinoma of the skin. Prior to surgery, patients received four treatments with the PD-1 drug Cemiplimab, followed by radical surgery with or without radiotherapy as appropriate.
The results showed that 50.6 per cent of patients had pathologically complete tumour disappearance, meaning that no tumour cells were visible at the time of surgery, and 12.7 per cent had pathologically mostly disappeared, meaning that less than 10 per cent of tumour cells were visible at the time of surgery.
"Such high efficacy of PD-1 monotherapy neoadjuvant has not been observed in any solid tumour to date! This could open up a new paradigm in the treatment of advanced resectable cutaneous squamous cell carcinoma." Neil Gross, lead author of the study and professor of head and neck surgery at MD Anderson Cancer Center, said. "We are still in long-term follow-up and I am looking forward to this new therapy improving patient survival outcomes, including quality of life."

Professor Neil Gross
Squamous cell carcinoma of the skin is a common skin cancer with a generally good prognosis, and most can be treated by dermatologists without the need for more aggressive treatment. However, as most are in the head and neck region, in rare cases they can grow and spread, invading the eyes, ears, nose and mouth. The current standard of care includes surgery and radiotherapy, which can lead to disfigurement and damage to vital organ function.
In contrast, in this study, preoperative immunotherapy allowed some patients to undergo less invasive surgery that could preserve more organ function.
In 2018, the US FDA approved cemiplimab for the treatment of patients with metastatic squamous cell carcinoma of the skin who are not eligible for curative surgery or radiotherapy. This trial validated its efficacy for use in the preoperative phase.
In terms of safety, common adverse reactions in this trial included fatigue, pimples, diarrhoea and nausea.
The research team will continue to follow up the subjects and further study the survival data. In the meantime, it is hoped to find answers to questions such as how many doses of treatment are better before surgery, which patients can safely avoid radiotherapy or even require surgery, which patients are more effective with immunotherapy, and so on.
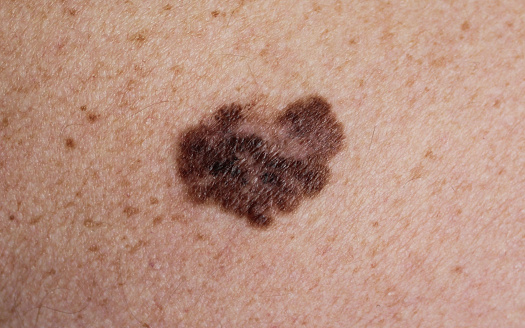
"I think the more important implication of the study is that it will improve the quality of survival for patients," said Professor Gross. "If radiotherapy can be avoided, or surgically removed to a lesser extent, then patients can keep their eyes, ears and nose."