St. Jude Children's Research Hospital, founded in 1962, is located in Memphis, Tennessee. The mission of St. Jude Children's Research Hospital is to improve the treatment of childhood illnesses and the means of prevention through research and treatment. No child will be denied treatment because of race, religion or a family's ability to pay.

St. Jude's has 3,600 employees, 69 beds in operation and treats approximately 8,600 patients each year. But we believe that even when battling a life-threatening illness, children should lead as normal a childhood as possible. That's why most of our patients are treated as outpatients and remain in one of our four housing facilities, which have nearly 300 rooms designed and managed by us specifically for families with children with cancer and other illnesses.

World-class medical contribution:
1966, world first to achieve sustained remission in patients with acute lymphoblastic leukaemia (ALL)
1977 First understanding of the lifelong progression of sickle cell disease

1984, Opening of the post-treatment clinic, the world's No.1 long-term follow-up clinic
1986 Using a risk-based approach to treatment, clinicians begin to reduce the amount of cranial irradiation in children with ALL
In 1988, ALL patients no longer received chemotherapy based on their size, but were treated according to each child's ability to break down the drugs in their bodies
In 1990, it was determined that children treated for malignant solid tumours had a low or negligible risk of developing secondary acute myeloid leukaemia (AML). haematocrit was found to counteract the life-threatening bone marrow depletion caused by the toxic effects of intensive chemotherapy;
1996, Dr Peter Doherty, Chair of Immunology, awarded the Nobel Prize in Physiology or Medicine (how the immune system recognises and kills cells infected by viruses)
1997, proved that bone marrow transplants from unrelated, genetically matched donors are as effective as bone marrow transplants from genetically matched siblings of patients in the treatment of childhood leukaemia
In 1998, by individualising the dose of chemotherapy, they were found to improve survival in children with ALL without causing excessive toxicity.
In 1999, a genetic defect was discovered that could cause secondary brain tumours in paediatric leukaemia patients

In 2003, a number of genes were discovered that could alter their characteristic pattern of activity levels in response to specific chemotherapies. These genes were identified in the leukaemia cells of children receiving ALL chemotherapy.
In 2005, certain traits inherited from parents may reduce the effectiveness of certain chemotherapeutic drugs in children with ALL. The discovery enabled clinicians to identify patients at high or low risk of relapse. Specific patterns of gene expression in leukaemia cells were found to be associated with resistance to their anti-leukaemia drugs. This finding helps explain why standard therapies fail to cure about 20 per cent of children with ALL.
In 2006, a 94% survival rate was reported for ALL patients using treatments that did not include radiation therapy.
In 2007, the LIFE study began to look at the long-term effects of cancer and its treatment. Drugs used for attention deficit disorder were found to help improve attention, social skills and behaviour in children treated for brain tumours and ALL. Elucidated why Philadelphia chromosome-positive ALL does not benefit from treatment.
Designated an NCI Comprehensive Cancer Centre in 2008, the first and only cancer centre focused on paediatric cancer to receive this distinction. A series of genetic mutations combine to cause BCR-ABL1-positive ALL, an aggressive and often fatal leukaemia. loss of the IKZF1 gene accompanies the transformation of chronic myeloid leukaemia to the acute, life-threatening stage.
In 2009, mutations in the IKAROS gene were identified that predicted a high likelihood of relapse in children with acute lymphoblastic leukaemia (ALL).
In 2010, the Paediatric Cancer Genome Project was launched in collaboration with the University of Washington to uncover why childhood cancers develop, spread and become drug resistant.
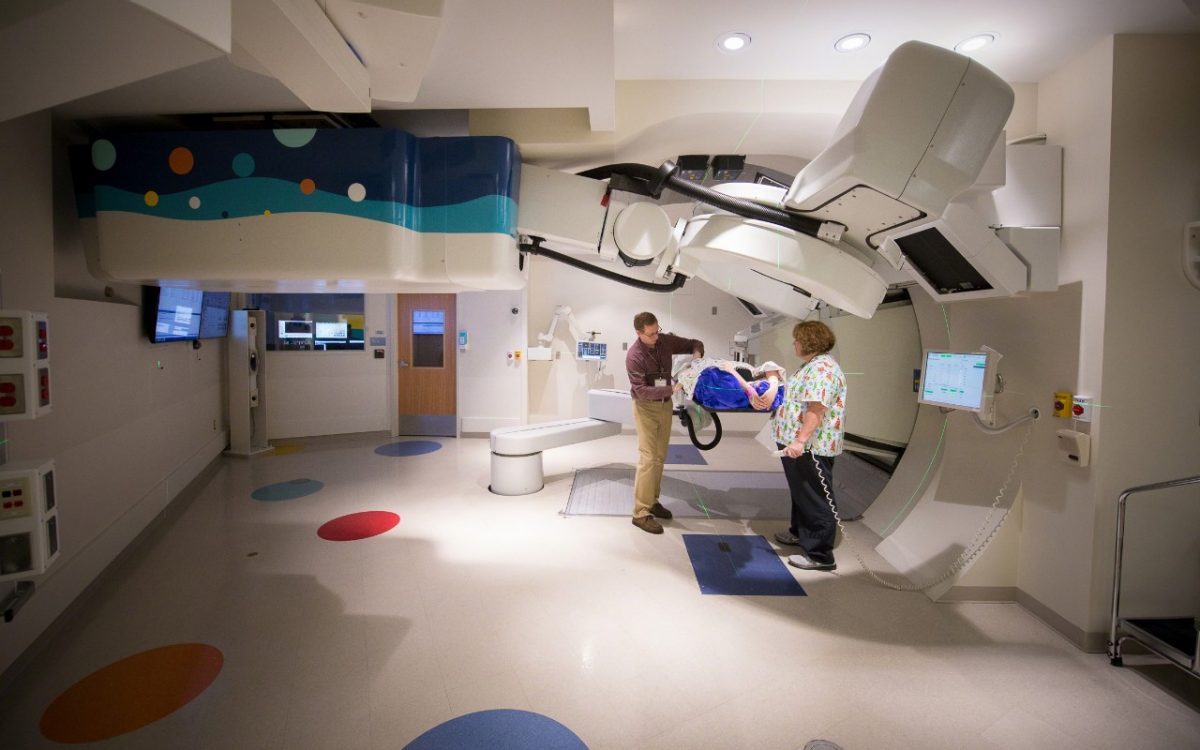
In 2015, the world's first proton therapy centre dedicated to children with cancer was opened.
The discovery that measuring leukaemia cells remaining in a patient's bone marrow in the first few weeks of treatment can help identify high-risk patients was the first to use a measure of residual leukaemia cells in the bone marrow, or minimal residual disease (MRD), to help guide treatment.
In 2017, it was discovered how immune cells called T cells are "exhausted" - unable to do their job of attacking invaders such as cancer cells or viruses. The discovery is important because patients treated with immunotherapy against cancer often fail to respond or have their disease return, and it has been suggested that these challenges may be due to T-cell exhaustion. The discovery provides a new avenue for more powerful and durable immunotherapies.
In 2018, a five-year collaboration with WHO was announced with a view to curing at least 60% of children worldwide with six common types of cancer by 2030, thereby transforming the way cancer is treated.
In 2019, the gene therapy SCID-X1 treats bubble boy disease (an ultra-rare and highly devastating genetic disease). By combining gene therapy and low-dose chemotherapy with leucovorin, the immune function of infants with this disease can be improved.
Leukaemia Centre
The only comprehensive cancer centre designated by the National Cancer Institute specifically for children. The Leukaemia Centre has a staff of 14 physicians. Leukaemia research has pioneered the world's approach to treating childhood leukaemia. Within weeks of starting treatment, approximately 98% of children with ALL are in remission. About 90% of these children can be cured. Patients are considered cured after 10 years of remission.

The survival rate for St. Jude's ALL patients is 94%, the best outcome for the disease worldwide. It was the first hospital in the US to remove cranial radiotherapy from ALL treatment without compromising survival rates. St Jude researchers have discovered an unexpected genetic alteration in a deadly childhood leukaemia, ETP-ALL, that could change the diagnosis and treatment of children with the disease.






