With more and more new cutting-edge drugs emerging, the standard treatment paradigm for breast cancer is now rapidly changing and the future is looking brighter for patients.

Data presented at the 2022 San Antonio Breast Cancer Symposium (SABCS) showed that in the Phase 2 TROPiCS-3 trial (NCT02), patients with hormone-receptor-positive, HER2-negative metastatic breast cancer treated with the ADC drug Sacituzumab Govitecan (i.e. Trodelvy) had a clear benefit in terms of overall survival (OS) and progression-free survival (PFS) with clear benefits.
*Progression-free survival (PFS): This refers to the time from the start of treatment to the time when disease progression or death is observed in patients with tumours. This metric is often used to determine drug efficacy.
"There is a lot of research data now that is changing the way we think about the treatment of hormone receptor-positive metastatic breast cancer," said Rugo, Professor of Medicine in the Department of Hematology and Oncology and Director of Breast Oncology and Clinical Trials Education at the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco.
"More importantly, it provides us with many new treatment options that will change the outcomes for the common population of hormone receptor-positive breast cancer patients."
The results of the TROPiCS-02 clinical study showed that Trodelvy significantly improved a range of efficacy indicators such as tumour remission rates and tumour control rates in patients (second column of data in the graph below) compared to other regimens chosen by physicians (TPC regimen), regardless of the patient's Trop-2 expression.
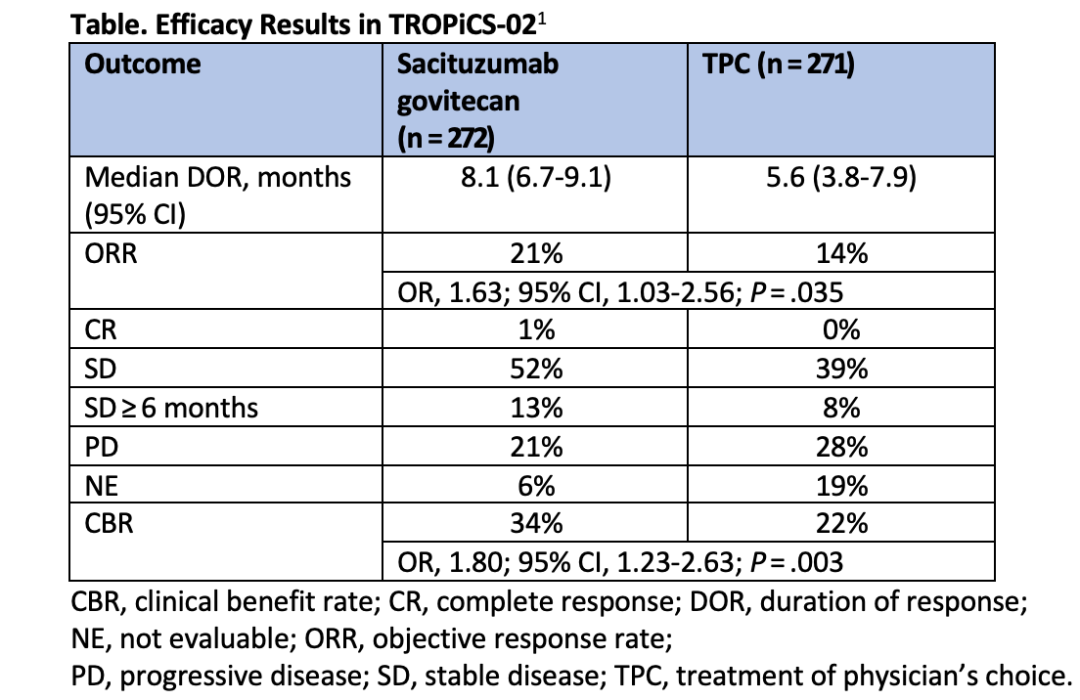
In the first interim analysis of the study, Trodelvy gave patients an overall survival of 11.2 months on average, compared to 4.4 months for patients in the control group who received the physician's choice regimen.
"Ninety-five per cent of the patients in the trial were Trop-2 positive, although the degree of expression was not the same," Rugo said. "Patients were divided into two categories, high and low Trop-2 expression, but the results showed that progression-free survival and overall survival improved in either category."
On February 3, 2023, the FDA approved Trodelvy for the treatment of unresectable locally advanced or metastatic breast cancer, requiring patients to be HER2 negative, hormone receptor positive, and receiving endocrine therapy as well as at least two late-stage systemic systemic-based therapies.
Breast cancer patients who have become resistant to endocrine therapy have very few late treatment options, but ADC drugs such as Trodelvy are making inroads into earlier stages of treatment. For example, Trodelvy is being evaluated for efficacy in the first-line treatment of PD-L1-positive triple-negative breast cancer.

"ADC drugs are an explosive area," says Rugo, "and we have DS-8201 for HER2-positive breast cancer treatment. Even breast cancers with low HER2 expression have the opportunity to significantly improve outcomes with this drug. There are many more clinical trials currently testing further drug combinations, such as the powerful combination of ADC drugs and immune checkpoint inhibitors."
Rugo said that the ADC drug DS-1062a, which targets Trop-2, and the ADC drug U3-1402, which targets HER3, are also currently in clinical trials.
The MONALEESA-2, MONALEESA-3 and MONALEESA-7 clinical trials together validated the efficacy of the CDK4/6 inhibitor Ribociclib in combination with endocrine therapy for the treatment of hormone receptor-positive, HER2-negative patients with advanced breast cancer.

Results showed that patients in the combination therapy group, compared to endocrine therapy alone, had significantly improved outcomes and substantially prolonged mean progression-free survival (44.2 months vs. 31 months).
In addition, the study results showed that patients who received CDK4/6 inhibitors after disease progression had better outcomes, while those who did not use CDK4/6 inhibitors after progression and opted for chemotherapy had relatively poor mean progression-free survival.
The Phase 2 AMALEE trial evaluated the optimal dose of Ribociclib for hormone receptor-positive patients, which should be 600 mg. A higher dose would give patients more adequate efficacy and, although it may increase neutropenia, would not affect adverse effects, including liver enzyme abnormalities, so patients should start at 600 mg if tolerated.
Capivasertib, another novel, highly potent pan-AKT kinase inhibitor, has also shown promising results in clinical studies. According to data from the Phase 2 CAPltello-291 clinical trial, the drug, in combination with fulvestrant, doubled mean progression-free tumour survival compared to the fulvestrant alone regimen (7.2 months vs. 3.6 months).Rugo said that within the next year, Capivasertib is expected to receive FDA approval for use after treatment with CK4/6 and aromatase inhibitors progressive breast cancer treatment.
In addition to the drugs mentioned above, clinical trials of other cutting-edge drugs such as oral selective estrogen receptor degraders and protein hydrolysis-targeted chimeras are also underway.

In conclusion, with the emergence of many promising cutting-edge breast cancer drugs, it is believed that the standard of care paradigm for breast cancer will change significantly in the future, and patients will benefit from this with improved prognosis and better control of their disease.






