As medicine continues to advance, there are a variety of good drugs and protocols available to fight cancer. Just recently, a study showed that the use of the NALIRIFOX regimen in patients with advanced pancreatic ductal adenocarcinoma who had not previously received treatment significantly improved overall patient survival.
The study in the phase III clinical trial NAPOLI-3 (NCT04083235) showed that the NALIRIFOX regimen, consisting of liposomal irinotecan (Onivyde), 5-fluorouracil/calcium folinic acid and oxaliplatin, significantly improved overall patient survival when compared to a regimen of paclitaxel combined with gemcitabine for the first-line treatment of patients with advanced pancreatic ductal carcinoma.
The researchers also found that the NALIRIFOX regimen increased the length of time that patients' tumours did not progress. In addition, the toxicities of the new regimen were consistent with those reported in previous phase 1/2 clinical studies.
In light of this, Ipsen announced plans to submit a supplemental new drug application seeking approval in the US for the treatment of patients with this type of pancreatic cancer.
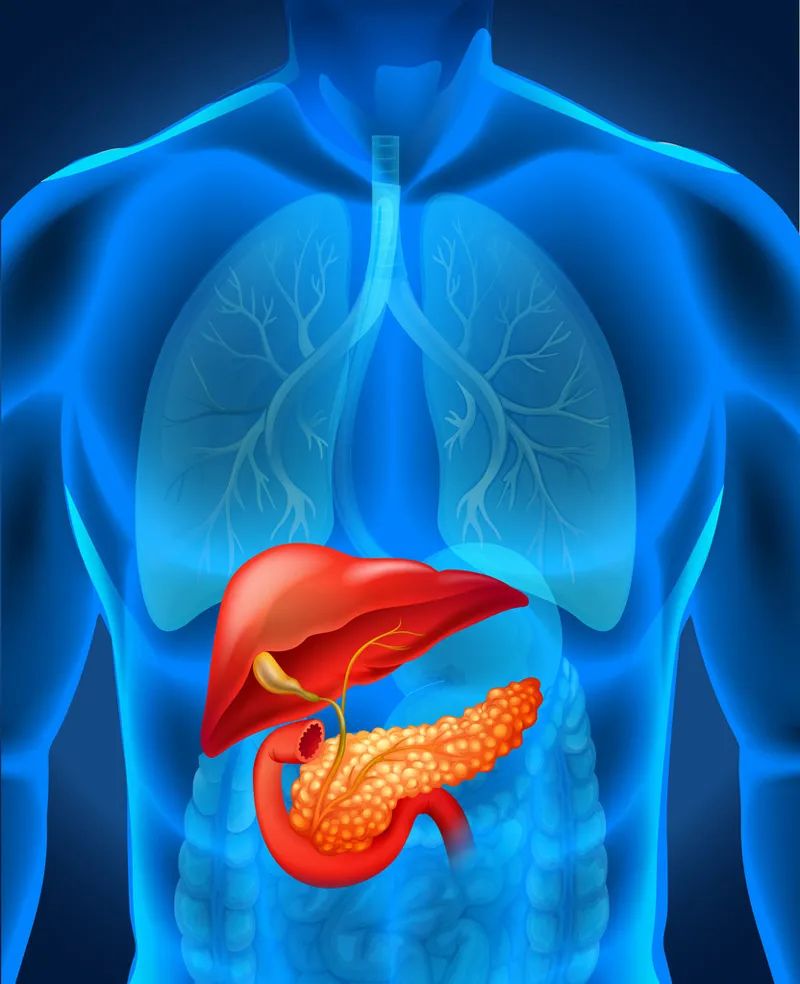
"The positive results of the NAPOLI-3 trial demonstrated that the addition of liposomal irinotecan extended survival in previously untreated patients with advanced pancreatic cancer compared to standard treatment." Howard Mayer, executive vice president and head of research and development at Ipsen, said in a press release.
The drug combination of liposomal irinotecan and 5-fluorouracil was previously approved by the FDA for the treatment of advanced pancreatic ductal carcinoma following gemcitabine treatment resistance. Supporting this approval is the Phase III NAPOLI-1 clinical trial. In this trial, the addition of liposomal irinotecan significantly prolonged patient survival compared to 5-fluorouracil/calcium folinic acid alone.
Data from an ongoing phase II study (NCT02551991) show that the NALIRIFOX regimen demonstrates encouraging anti-tumour activity in the treatment of locally advanced or advanced pancreatic ductal carcinoma in the pancreas. Researchers will proceed to further evaluate the safety and efficacy of this regimen in the patient population.
Patients with pancreatic cancer recruited for the NAPOLI-3 clinical study will be randomly assigned 1:1 to either the liposomal irinotecan arm or the paclitaxel combined with gemcitabine arm, and treatment will continue until patients progress, become intolerant to dosing or withdraw voluntarily.
In addition to the primary indicator of survival, the researchers will look at the incidence of both regimens in terms of tumour progression-free survival, objective tumour remission rates, patients' quality of life assessment, adverse reactions in the treatment primaries, and serious adverse events.
"As the prognosis for patients diagnosed with pancreatic cancer is generally very poor, we plan to submit these new findings to regulatory authorities and if the regimen can be approved, it could provide an important new treatment option for patients with pancreatic cancer."
Liposomal Irinotecan Onivyde, a liposomal injection of the pancreatic isomerase inhibitor Irinotecan, has a long-lasting presence in the systemic circulation. Thanks to its cutting-edge liposome coating, liposomal irinotecan has a stronger penetration effect into cancer cells, a lower half-life and a longer duration of action.