Afamitresgene Autoleucel, also known as Afami-cel or ADP-A2M4, is a novel TCR-T cell immunotherapy that has produced durable and promising therapeutic results in patients with advanced synovial sarcoma or mucinous/round cell liposarcoma.
Based on results from Cohort 1 of the ongoing Phase 2 SPEARHEAD-1 trial (NCT04044768), Afami-cel, a novel TCR-T cell therapy, resulted in an overall remission rate (significant tumour shrinkage or disappearance) of 38.6% in 44 heavily treated patients with synovial sarcoma, with a mean duration of tumour shrinkage of 50 The average duration of tumour shrinkage in patients was 50 weeks, with the duration of tumour shrinkage even reaching 122 weeks in patients enrolled with good results.
"With these study data, we are excited about the clear potential of afami-cel therapy for the treatment of synovial sarcoma. Synovial sarcoma is a very difficult cancer to treat and the needs of patients are far from being met," said Dr Elliot Norry, Chief Medical Officer of Adaptimmune, in a press release. "We are excited to include this research data in our upcoming Biologics License Application (BLA) submission."
Adaptimmune plans to use the data to support Afami-cel in a subsequent BLA application, which is expected to be completed by mid-year 2023.
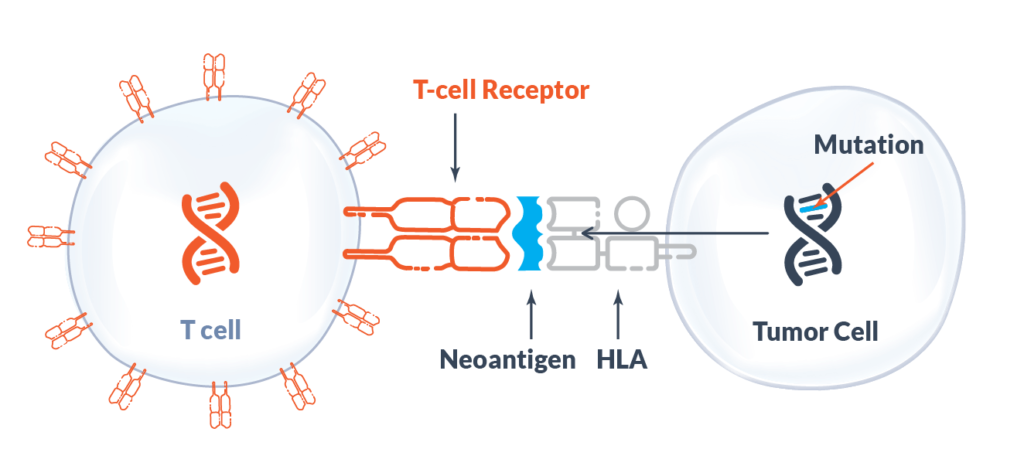
afami-cel, a TCR-T therapy, targets a target in tumours called MAGE-A4, which is highly expressed in a variety of solid tumours, including synovial sarcoma, and plays an important role in tumour development. Through Adaptimmune's SPEAR platform, cancer cell-specific 'tags' can be identified and TCRs that bind to these 'tags' can then be screened and genetically engineered to help activate the T cells' cancer-fighting power. The TCRs are then genetically engineered to help activate the T-cell resistance.
At this year's ASCO annual meeting, summary data from the 69 patients in the study were previously presented: the average percentage of patients whose tumours shrank or disappeared was 36.2%, and the average length of tumour shrinkage was 52 weeks. Apparently, the before-and-after data were similar and largely consistent.
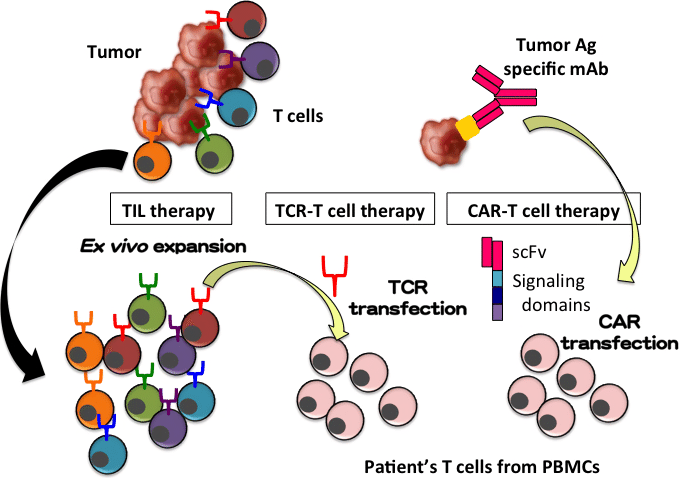
Adaptimmune says these data show that afami-cel therapy, drives the tumour infiltrative capacity of activated and proliferative human immune T cells and helps to transform the tumour microenvironment, which is otherwise immunosuppressive, into one that promotes the anti-tumour capacity of immune cells.
With respect to the toxicity of the therapy, which primarily includes cytokine release syndrome and reversible haematological toxicity, the safety profile is acceptable.
Among all patient groups evaluated, female patients appeared to have a higher response rate. Also, patients with higher MAGE-A4 expression and a lower disease burden at the start of treatment had better results.
"We are at an exciting and important time for patients with synovial sarcoma, a cancer for which there have long been few innovative treatment options," said Brian A. Van Tine, MD, professor of medicine and pediatrics at Washington University School of Medicine in St. Louis. "The results of this trial give us great hope that more patients with synovial sarcoma will be better treated in the near future."