Pancreatic cancer has long been known as the "king of cancers" due to its poor prognosis. However, a recent study showed that adding Mitazalimab to the modified FOLFIRINOX regimen resulted in disease control in over 90% of patients with metastatic pancreatic cancer, with 52% of patients experiencing significant tumour shrinkage!
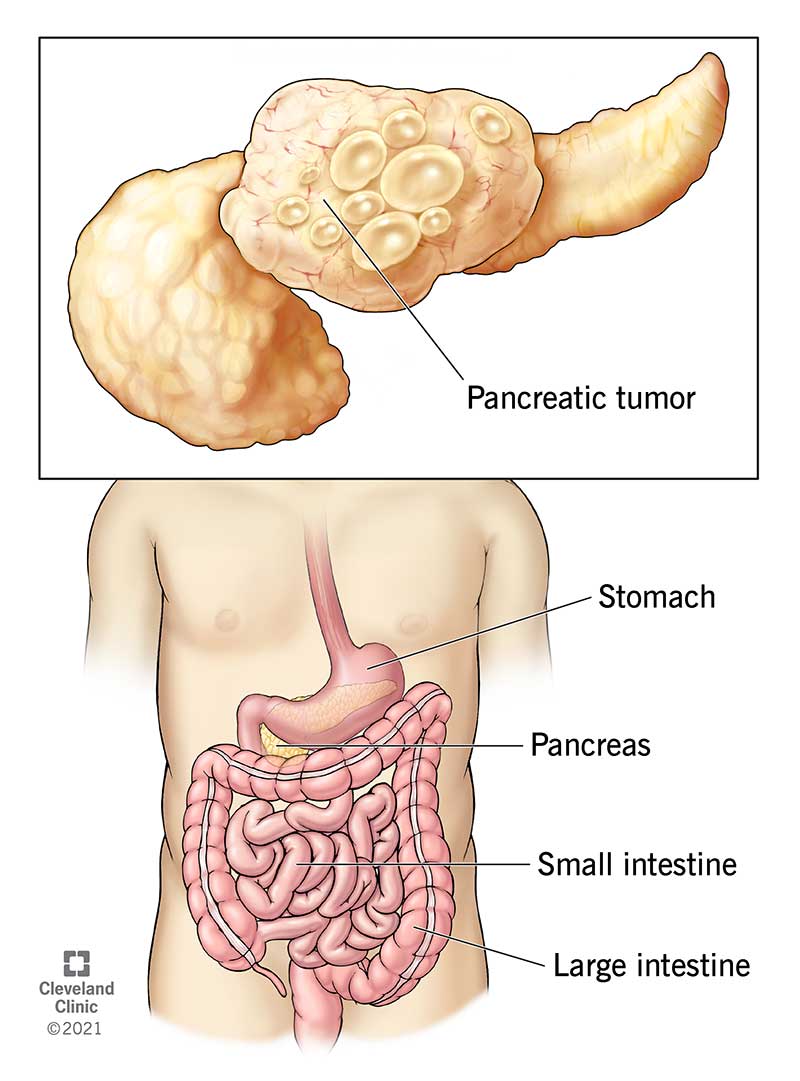
The Phase 2 OPTIMIZE-1 study recently showed that adding Mitazalimab to a modified FOLFIRINOX regimen resulted in significant tumour shrinkage in 52% of 23 patients with metastatic pancreatic cancer. This compares with 31.6% of patients with pancreatic cancer whose tumours shrank substantially on the FOLFIRINOX regimen alone in the previous study. Thus, the addition of Mitazalimab significantly improved the efficacy of the treatment.
Other data also showed that the Mitazalimab + modified FOLFIRINOX regimen resulted in more than 90% of patients having controlled disease without further progression. Safety data were also consistent with previous ones, with good tolerability.
Based on these results, Mitazalimab's development organisation plans to discuss with European and US regulatory authorities ways to fast-track approval.
Mitazalimab is a human-derived CD40 agonist IgG1 antibody that targets CD40 on the surface of dendritic cells, a type of cell in the immune system that detects cancer cells. Mitazalimab can rapidly initiate cancer immunity by recruiting dendritic cells, leading to the initiation and activation of tumour-specific T cells. In addition, CD40 agonists also promote the entry of T cells and chemotherapeutic agents into the tumour, further enhancing the ability to kill the tumour.
OPTIMIZE-1 is a multi-centre trial designed to evaluate the effectiveness of this monoclonal antibody Mitazalimab, which targets CD40, in combination with modified FOLFIRINOX chemotherapy, in patients with untreated metastatic pancreatic cancer.
Safety data obtained from the Phase 1b portion of the OPTIMIZE-1 trial were presented at ASCO 2022, where five patients were treated with a 450 µg/kg dose of Mitazalimab with milder grade 1-2 side effects including fever, muscle pain and nausea. No patients experienced dose-limiting toxicity, and no patients discontinued or had their dose reduced due to side effects.
"We are pleased with the positive clinical activity observed in the OPTIMIZE-1 study and believe that Mitazalimab in combination with chemotherapy for pancreatic cancer is worthy of continued study as there are still many unmet needs in this patient population and we will continue to recruit, treat and follow up patients to further determine survival benefit. " said the trial's principal investigator.